The removal of a genetic roadblock could improve the efficiency of converting adult cells into stem cells by 10 to 30 times, report scientists from The Methodist Hospital Research Institute and two other institutions in the latest issue of Cell.
“The discovery six years ago that scientists can convert adult cells into inducible pluripotent stem cells, or iPSCs, bolstered the dream that a patient’s own cells might be reprogrammed to make patient-specific iPSCs for regenerative medicine, modeling human diseases in petri dishes, and drug screening,” said Rongfu Wang, Ph.D., Principal Investigator and Director of the Center for Inflammation and Epigenetics. “But reprogramming efficiency has remained very low, impeding its applications in the clinic.”
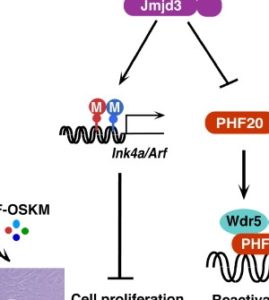
Wang and his group identified a protein encoded by the gene Jmjd3 (also called KDM6B) as a roadblock in the stem cell conversion process. Jmjd3 is known to be involved in many biological processes, including the maturation of nerve cells and immune cell differentiation.
Wang and his team are the first to identify Jmjd3’s role in inhibiting the reprogramming process. They found knockdown or deletion of Jmjd3 in young mouse fibroblasts was enough to greatly enhance reprogramming efficiency.
“Our findings demonstrate a previously unrecognized role of Jmjd3 in cellular reprogramming and provide molecular insight into the mechanisms by which the Jmjd3-PHF20 axis controls this process,” said Helen (Yicheng) Wang, co-principal investigator.
In investigating Jmjd3 and its role in iPSC reprogramming, Wang’s team found Jmjd3 has two previously unknown functions — it helps regulate cell growth and cellular aging and Jmjd3 deactivates another nuclear protein, PHF20. The scientists learned during the study that PHF20 is required for cellular reprogramming, because cells without PHF20 failed to generate iPSCs.
“So when it comes to increasing iPSC yields, knocking down Jmjd3 is like hitting two birds with one stone,” Rongfu Wang said.
Jmjd3 may not be the only genetic roadblock to stem cell conversion.
“Removal of multiple roadblocks could further enhance the reprogramming efficiency with which researchers can efficiently generate patient-specific iPSCs for clinical applications,” Wang said.
Also contributing to this work were Wei Zhao, Qingtian Li, Stephen Ayers, Yifeng Gu, and Qingyuan Zhu (TMHRI), Zhong Shi (Baylor College of Medicine), and Yidong Chen (Univ. of Texas Health Sciences Center at San Antonio). The research was in part supported by the National Cancer Institute (NIH) and the Cancer Prevention Research Institute of Texas.
